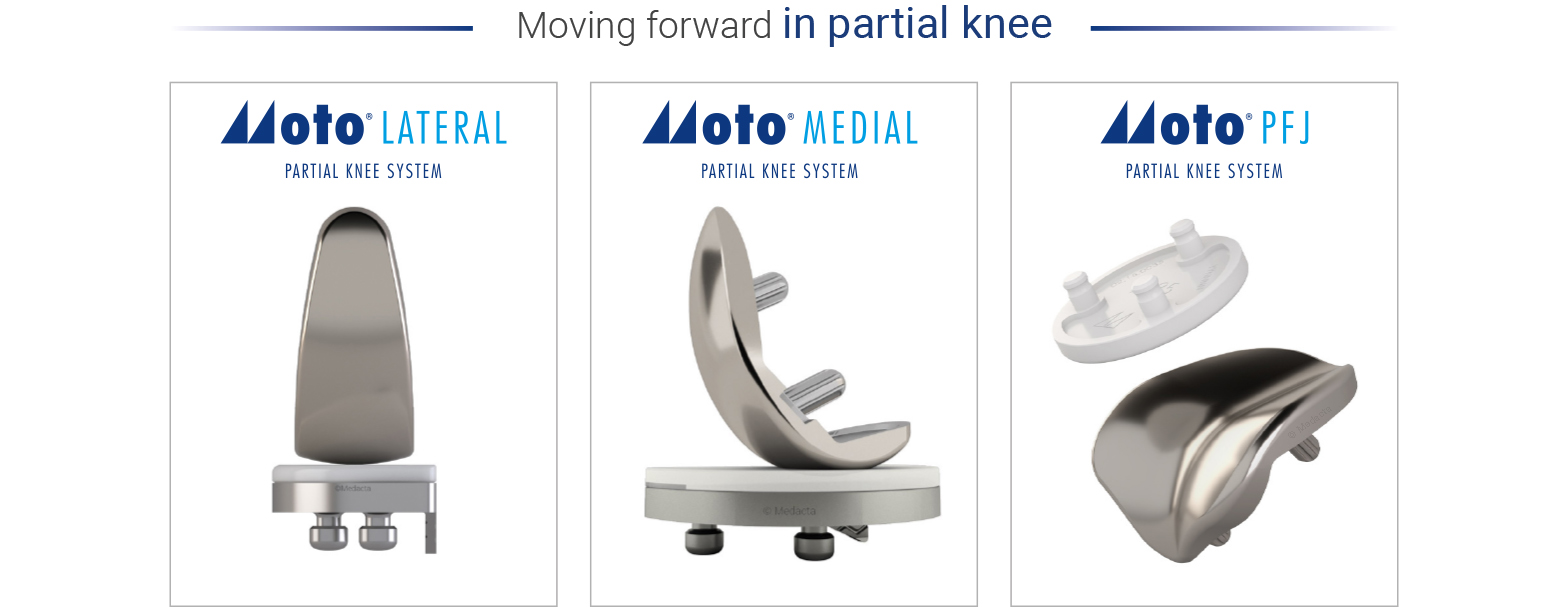
"It is exciting to have this new MOTO Patellofemoral Joint Replacement system available from Medacta,” says Akbar Nawab, M.D. “The instrumentation is very well designed, avoiding the need to disrupt the femoral canal. The ability to adjust the anterior cut in every degree of freedom allows the surgeon to address even the most dysplastic trochlear anatomy. The lateralized trochlear groove of the femoral component was critical in this case, avoiding the need for a lateral retinacular release or lengthening. The different patellar sizes and thicknesses give the surgeon many options. The MOTO Patellofemoral system is a great addition to the MOTO partial knee replacement family. I am thrilled to have implanted the first MOTO PFJ. "
With this final addition to the comprehensive MOTO Partial Knee System, already including MOTO Medial and MOTO Lateral, surgeons can now provide their patients with a full range of partial knee arthroplasty options to best treat their specific needs. The MOTO System was designed to accommodate the individual anatomy in order to achieve optimal coverage and fit, and to provide correct and individualized balance and alignment at every step of the procedure with the potential of decreasing the incidence of loosening and progression of disease.
To provide surgeons with the possibility of tailoring the implant choice to the patient’s needs for partial, total and revision knee replacement, Medacta has developed advanced material options:
SensiTiN™, a ceramic-like coating designed for reduced metal ion release and E-CROSS
®, a highly crosslinked UHMWPE blended with Vitamin E, a powerful antioxidant that improves oxidation resistance, which are available for MOTO and
GMK® Sphere tibial inserts.
MOTO System is also supported by Medacta's
M.O.R.E. Institute, which provides tailored high-level educational pathways through an international network of partial knee expert surgeons. With Medacta the surgeon is never alone.

